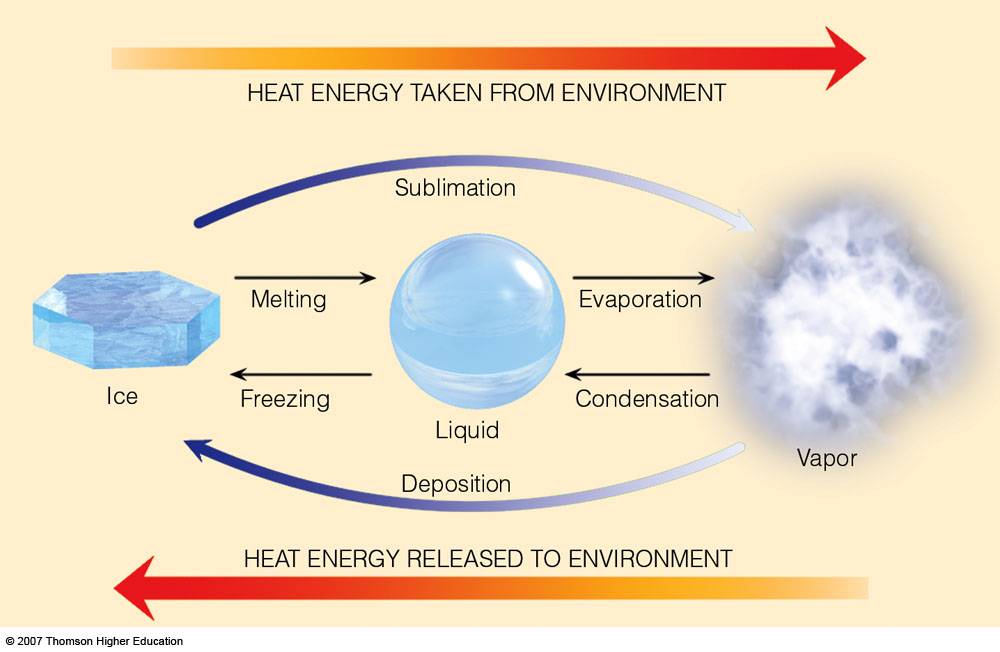
Latent Heats
When a substance changes from one state to another, latent heat is added or released in the process. Consider the water substance:
ice --> vapor , latent heat of sublimation is added
Q: how much? ANSWER
vapor --> ice, latent heat of deposition is released
Q: how much? ANSWER
Q: Is latent heat a big deal in the atmosphere? ANSWER
QUESTION FOR THOUGHT:
a. In a hurricane, if 100 grams of water vapor condense into water every second, how many calories of heat are released into the atmosphere in one day? How much additional heat per day would be released if the water then froze into ice particles?
b. If the mass of a dry air parcel in a thunderstorm is 50 kg, how much cooler will the parcel be if 40 grams of ice sublimates into water vapor within the parcel?