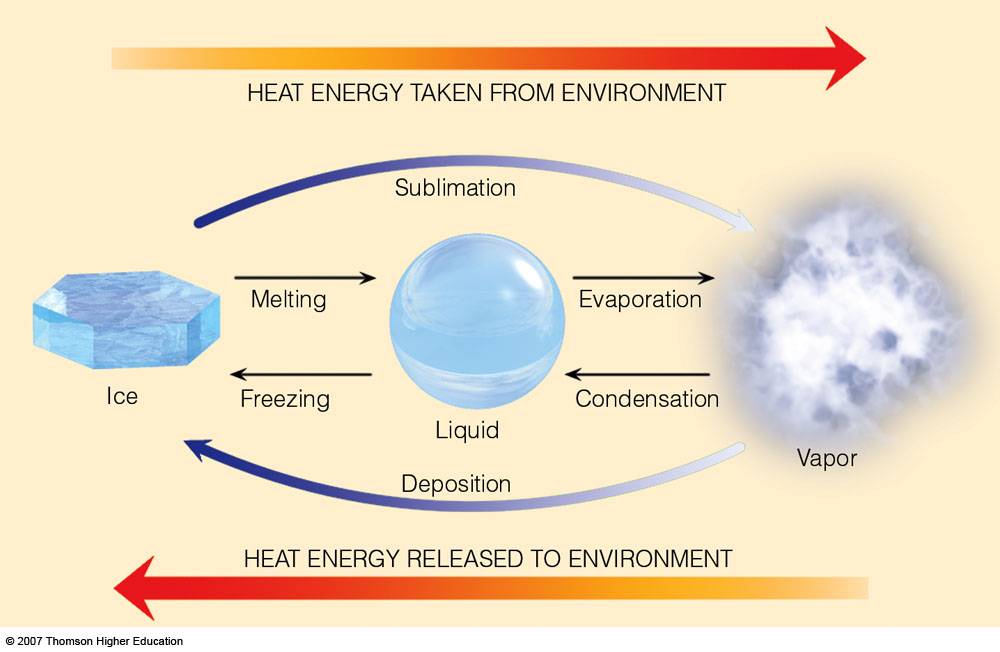
Latent Heats
Consider the water substance:
liquid --> vapor, latent heat of evaporation is added (about 600 cal per gram)
Q: What are some real-world examples of this process????
vapor --> liquid, latent heat of condensation is released
liquid --> ice, latent heat of freezing is released (about 80 cal per gram)
ice --> liquid, latent heat of fusion (melting) is added