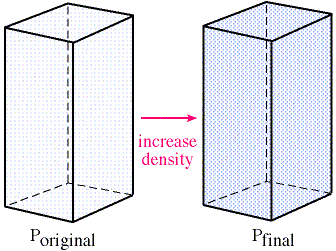
Pressure changes due to density variations
Recall the ideal gas law:
P = CrT
if T is constant, then P can increase by increasing the density of the gas -->>
conversely, if the density decreases, so does the pressure
Q: What happens if you change the temperature of the air column?