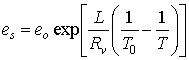
Homework for Chapter 4 - Atmospheric Moisture
SHOW ALL WORK , CIRCLE THE CORRECT ANSWER, PLEASE BE NEAT AND STAPLE YOUR HOMEWORK!
ALSO, PLEASE USE A SPREADSHEET FOR ALL GRAPHS
Follow the Problem solving steps discussed in class
1. It can be shown that the saturation vapor pressure (es) depends only on temperature:
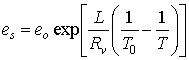
where eo = 6.11 hPa and To = 273 K are constant parameters. Rv is the gas constant for water vapor and is equal to 461 JK-1kg-1. L is the latent heat of evaporation if water is present or the latent heat of deposition if ice is present. T is the temperature in Kelvin
a. For water, create a plot of es versus temperature for T=250 K to 273 K. Make sure es is on the y axis and temperature is on the x axis. How does es depend on temperature? exp refers to the exponential function.
b. On the same graph, plot es versus temperature for ice.
c. Is es larger for ice or for water?
2. Given a parcel of air having a temperature of 35 degrees C and relative humidity of 95% find:
a. saturation vapor pressure
b. vapor pressure
d. If the temperature of the air increased to 40 degrees C will the air feel more or less humid? Explain.
3. Explain how and why each of the following will change as a parcel of air with an unchanging amount of water vapor rises, expands, and cools: (a) absolute humidity; (b) relative humidity; (c) actual vapor pressure; (d) saturation vapor pressure
4. Where in the U.S. would you go to experience the least variation in dew point from January to July?
EXTRA CREDIT: Given two parcels, one is dry containing 78% N2, 21% O2, and 1% AR while the other is moist, containing 78% N2, 21% O2, 0.5% AR, and 0.5% H2O. Show that the dry parcel has a larger density than the moist parcel.