Determining Past Climate Change - Oxygen Isotopes
- normal oxygen contains 8 protons, 8 neutrons (O16)
- a small fraction (one in a
thousand) of oxygen atoms contain 8 protons, 10
neutrons (O18)
- this is an isotope of oxygen and is heavier than O16
- O16 will evaporate more readily than O18 since it is lighter
- Hence, during a warm period, the relative amount of O18 will increase in the ocean waters since more of the O16 is evaporating
- Hence, looking at the ratio of O16 to O18 in the past can give clues about global temperatures.
- Ice cores from glaciers can also give you similar information
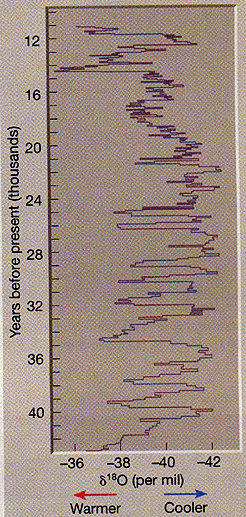
image adopted from The Amosphere by Lutgens and Tarbuck, © 1998 by Prentice-Hall, Inc